Research
Microfluidics for Biomedicine

Our main objective is to advance the understanding of microscale fluid flow, specifically to facilitate the development of integrated micro/nanosystems for applications in biology and medicine.
Our research encompasses a broad range of disciplines, attracting students and researchers from diverse backgrounds. For further information, please reach out to Professor Chung, who can provide more details about our work.
Main research Topics
1. Gene Editing and Cancer Immunotherapy via Intracellular Delivery: |
|
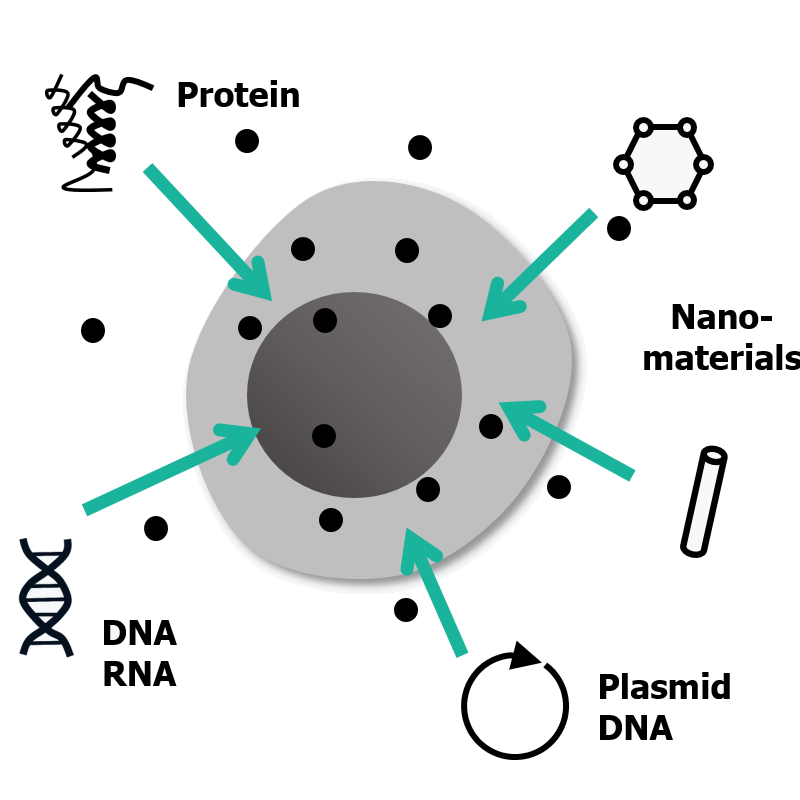 |
The successful introduction of biomolecules and nanomaterials into cells plays a crucial role in various fields, spanning from fundamental biology to clinical applications. At our lab, our primary goal is to pioneer innovative microfluidics-based intracellular delivery platforms. These platforms are designed to effectively deliver a wide range of nanomaterials into primary cells that are typically challenging to transfect, all without the need for carriers or external apparatus.
Moreover, we are capitalizing on these platforms to advance cancer immunotherapy, regenerative medicine, and genome editing. For example, we are actively working on the development of non-viral transfection technologies to enhance cell-based immunotherapies. Additionally, we are dedicated to establishing cutting-edge microfluidic strategies for next-generation genome editing techniques such as base and prime editing. |
2. Microfluidics for LNP production and screening: |
|
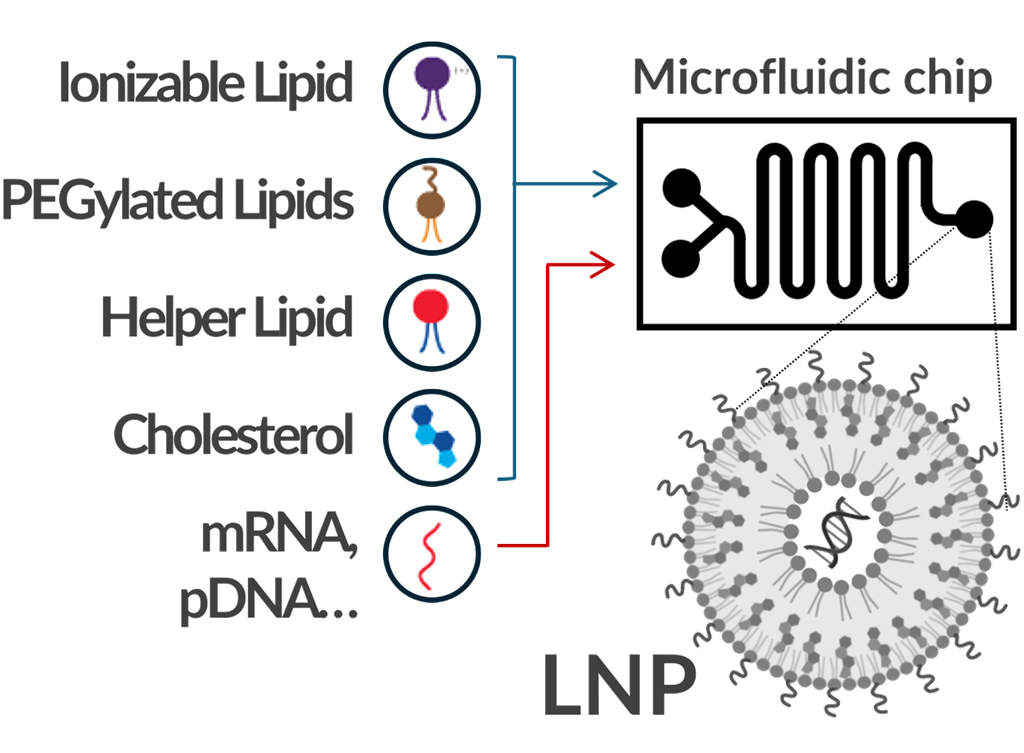 |
Lipid nanoparticles (LNPs) have gained prominence in the pharmaceutical industry as promising non-viral delivery vehicles, offering a safe and efficient method for mRNA delivery. Expanding their use beyond mRNA-based therapeutics requires optimizing the ratios of ionizable lipids, helper lipids, cholesterol, and PEG-lipids to enhance delivery efficiency. This optimization process must also be tailored to the specific cell type, cargo, and intended application. However, such efforts are often extremely time-consuming and labor-intensive. To overcome these challenges, we are developing microfluidic platforms that enable the rapid and cost-effective production and screening of LNP formulations. |
3. Single-cell Mechanotyping: |
|
 |
The mechanical properties related to cytoskeletal structures, such as cell deformability, have emerged as valuable label-free biomarkers for characterizing cell states and properties. Our primary focus is on the development of advanced platforms for real-time, multiplexed, high-throughput (>1K cells/sec), and label-free measurement of cell deformability. These platforms are designed to serve as screening and sorting tools, enabling quantitative assessment of cell deformability for cancer diagnosis. |

